Now Reading: Toxic Syrups Alert: Why WHO Warns of Unregulated Export Risks in India
-
01
Toxic Syrups Alert: Why WHO Warns of Unregulated Export Risks in India
Toxic Syrups Alert: Why WHO Warns of Unregulated Export Risks in India
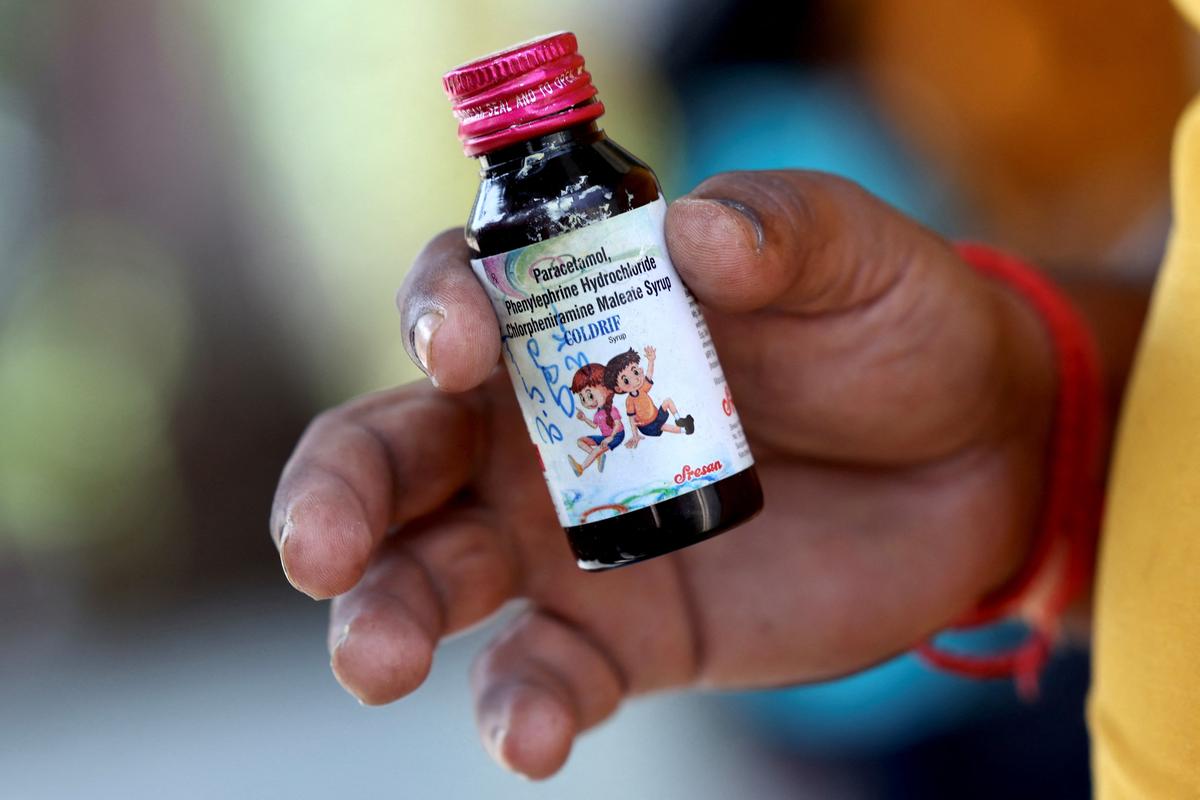
The World Health Organization has raised an alarm over the possibility that contaminated cough syrups could be exported from India through unregulated channels. With recent reports of child fatalities linked to such syrups in parts of India, the warning is especially serious for smaller towns and border areas where oversight may be weaker. The question now is: can regulation keep up before more harm is done?
The health scare in focus
Some syrups sold in India were found to contain dangerous substances like diethylene glycol (DEG) and ethylene glycol—chemicals used in antifreeze and industrial solvents. Even small amounts can be toxic, particularly for children. The WHO said that while there is no confirmed record of these products being exported, the risks via informal networks cannot be ignored.
India’s regulatory response
Indian drug authorities have responded by confirming that no contaminated syrups officially left India. Still, they have ordered bans on the implicated products, launched sample testing, and urged stricter market surveillance, especially in unregulated areas. Many states have already issued alerts to pharmacies, hospitals, and distributors.
Why smaller towns are vulnerable
Tier-2 and Tier-3 cities often depend on suppliers with weaker compliance, weaker inspections, and less awareness of national drug safety alerts. In remote areas, the supply chain is longer, and the possibility of counterfeit or smuggled drugs increases. Local clinics or chemists may be the only access people have to medicines, making their risk exposure higher.
Challenges in enforcement
Tracking informal exports is notoriously difficult. Products sold off-the-books or across state borders may leave no formal record. Moreover, local drug inspectors and enforcement agencies often lack resources. Even when a ban is imposed, replacing banned products and ensuring alternate safe medicines reach every corner remains complex.
What citizens should watch and demand
People should ask chemists to show licensing and batch details. Be wary of unusually low prices or medicines without clear packaging or labelling. Health workers and NGOs in smaller cities can push for training and awareness campaigns. At policy level, citizens must demand stronger regulation, better capacity for drug inspection, and swift action on violations.

























