Now Reading: WHO Sound Alarm on 3 Cough Syrups After Child Deaths in Madhya Pradesh
-
01
WHO Sound Alarm on 3 Cough Syrups After Child Deaths in Madhya Pradesh
WHO Sound Alarm on 3 Cough Syrups After Child Deaths in Madhya Pradesh
A health alert has been issued over three cough syrups consumed by children who later died in Madhya Pradesh, prompting urgent scrutiny of drug safety and regulatory gaps across India. As panic spreads beyond metros, parents and health authorities in Tier-2 cities are scrambling for clarity. This is not just a medical story—it’s a wake-up call for oversight, trust and accountability in our pharmaceutical system.
What Happened: The Crisis Unfolds
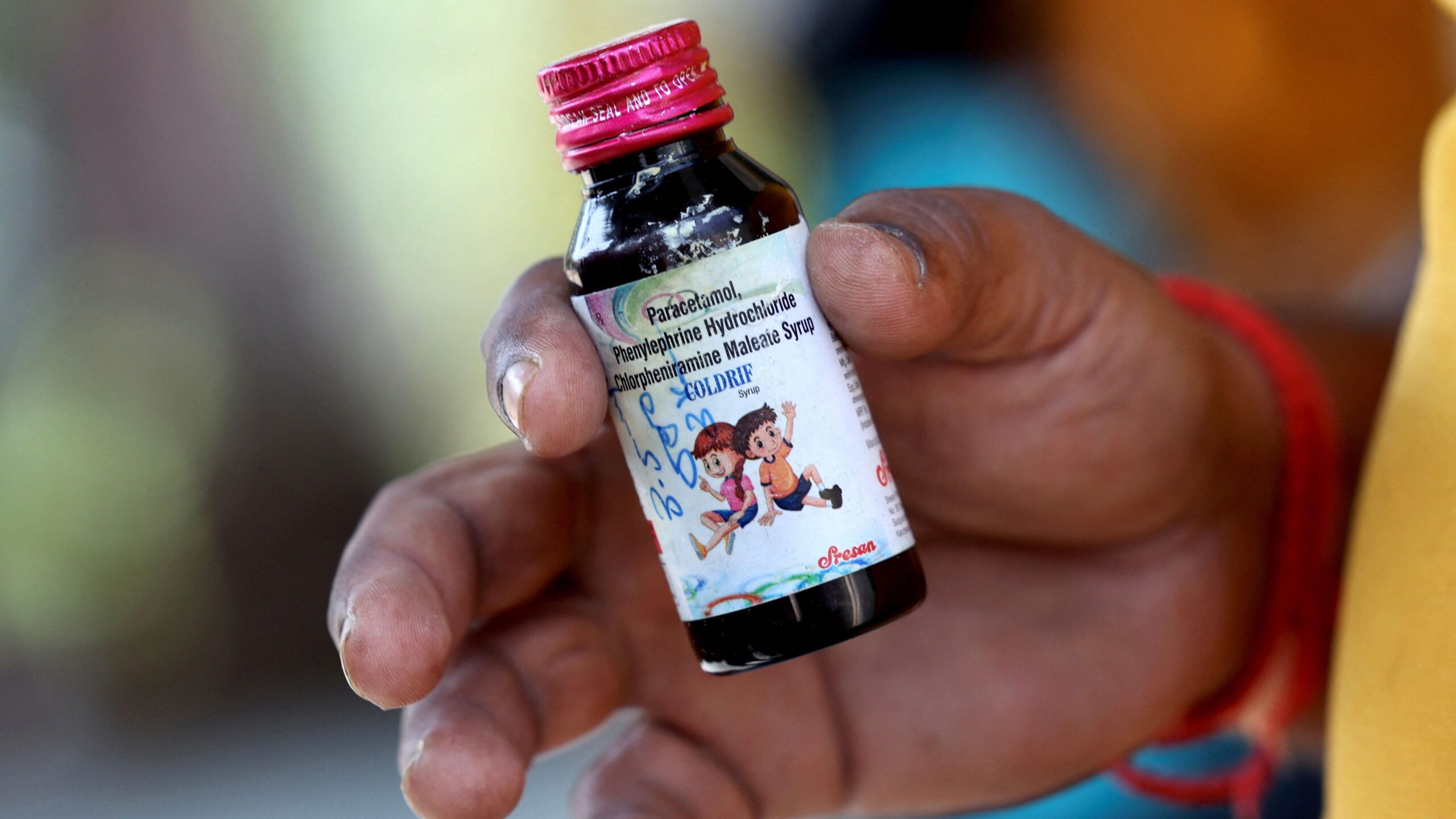
Several children under the age of five in Chhindwara district fell gravely ill and passed away after reportedly ingesting a cough syrup. The World Health Organization flagged three specific syrups—Coldrif, Respifresh TR and ReLife—as potentially contaminated. Investigations suggest the presence of diethylene glycol, a toxic solvent far above permissible limits, in these products.
The affected syrups have been immediately removed from sale, and authorities across states have begun recalls. The manufacturer whose product was linked to deaths has come under intense investigation, and regulatory agencies are facing pressure to explain how substandard medicines reached children in the first place.
Regulatory and Oversight Challenges
India’s drug regulatory framework is extensive on paper, but enforcing standards is uneven. In many states, drug inspectors are understaffed and laboratories overburdened. Local manufacturers in smaller towns often escape frequent audits. When medicines meant for children fail at basic safety checks, it points to systemic weaknesses—not just a single corporate failure.
Another problem is that domestic drug products often receive less scrutiny than exports. Some syrups flagged earlier in other countries (like Gambia or Uzbekistan) were also manufactured in India. This raises questions about why safety lapses are repeatedly discovered only after tragedies.
The Ripple Effect in Tier-2 and Beyond
In Tier-2 cities, distrust of healthcare systems is already fragile. When news breaks of medicines harming children, rumors and fear spread fast. Many parents will hesitate to administer any cough syrup—even safe ones—without strong assurance. Clinics and pharmacies in smaller towns must now respond not just with medicines but with credibility and transparency.
Local health officials are also under pressure. In towns with limited lab facilities, confirming contamination takes time and resources. That delay can cost lives or heighten fear in communities already distant from advanced medical centers.
What Needs to Happen Now
First, all states must coordinate fast recalls and public warnings. Parents should be told clearly which brands and batches to avoid. Medical practitioners must pause prescribing similar combination syrups—especially in children—until safety is confirmed.
Second, drug regulators need to conduct surprise checks, inspect manufacturing units, and audit supply chains, especially in remote areas. Transparency of results should be public to rebuild trust.
Third, laboratories in Tier-2 and Tier-3 regions must be strengthened—so samples from local chemists and small hospitals can be tested quickly. This avoids delay and local panic.
Fourth, legal action must follow. When negligence or misconduct contributes to deaths, accountability is not optional. That includes corporate directors, regulators who ignored risk, and middlemen in the supply chain.
The Bigger Picture: Trust, Health, Accountability
Medicine is among the most intimate contracts between state and citizen: you swallow, believing it will heal, not harm. When that trust is broken, the damage is deeper than just death. In India—where many already struggle with healthcare access—the emotional and social cost can be devastating, especially in smaller towns.
This incident must not be treated as a freak accident. It highlights persistent gaps in regulation, inspection, and enforcement. For true reform, systems must change—not just remove batches from shelves after disaster strikes.
Conclusion
The deaths linked to toxic cough syrups are a tragedy that demands systemic reckoning. For India’s health system—especially in its smaller cities—it’s time to prioritize safety over cost, regulation over laxity, and accountability over excuses. Only then can families trust that the medicine given to heal will never be the one that harms.